A Water Molecule Is Best Described as
Molecules breaking into ions. The attraction of water to substances other than water is known as _____ Adhesion.
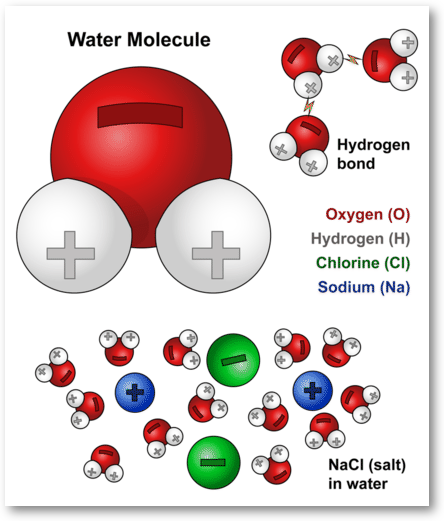
Water Molecules And Their Interaction With Salt U S Geological Survey
A water molecule is best described as _____ Two hydrogens covalently bonded to a single oxygen.

. Masaru Emoto performed a series of experiments observing the physical effect of words prayers music and environment on the crystalline structure of water. A mingling of molecules andor ions. Humans and bacteria share a common genetic code.
The molecule that is best described as glucose has the chemical formula C6H12O6. Humans and bacteria share a common genetic code. What would a good student reply.
The following molecule is best described as a. The Water Molecule Is Best Described as Ne_Weston166 April 13 2022 We dont longer stand behind the arguments and the validity of these claims Through the 1990s Dr. 1 N2O 2 SO 2 3 CaCl 2 4 HCl ___ 28 What type of bonding is found in the.
Since oxygen is more electronegative as compared to hydrogen atoms the shared electrons are attracted towards the oxygen atom. Humans should help in the conservation of other animal species. This molecule is best described as 1 nonpolar with nonpolar covalent bonds 2 polar with nonpolar covalent bonds 3 nonpolar with polar covalent bonds 4 polar with polar covalent bonds ___ 27 Which compound is ionic.
What is the diatomic nitrogen molecule best described as. The following molecule is best described as a. Ionic bonds polar covalent bonds non-polar covalent bonds hydrogen bonds When a pair of electrons is shared between two atoms the bond is called.
View the full answer. Water molecules attracted to other water molecules demonstrates the property of _____ Cohesion. The Water Molecule Is Best Described as By Da_Meadow91 09 Apr 2022 Post a Comment Water Facts Properties Structure Compounds Summary Lesson Summary Water And Life Article Khan Academy Water Molecules And Their Interaction With Salt U S Geological Survey.
In addition diffusion Monte Carlo DMC results are presented for several slab models as well as for the. Water is best described as A good solvent capable of dissolving non-polar molecules. Water H 2 O likewise has 2 H atoms associated with each O although the hydrogen atoms are relatively free to move to other oxygen atoms the overall behaviour makes more sense when considered as discrete water molecules in reality water behaves as though a variable number of H 2 O joined together but the units are joined by much weaker and less specific bonds.
Humans are controlled by forces beyond our understanding. In a beaker of water the bonds within a water molecule can best be described as. Dissolving is best described as.
A change from a solid to a liquid. The unequal sharing of electrons within a water molecule makes the water molecule _____. What electral change of a molecule of water is.
When dealing with polarity of a molecule one needs to consider the electronegativity how much a particular atom wants electrons. ___ 26 The diagram below represents a water molecule. The interaction of a water molecule with the 100 surface of MgO as described by cluster models is studied using MP2 coupled MP2 MP2C and symmetry-adapted perturbation theory SAPT methods.
Water is sometimes called the universal solvent. Is a water molecule described as being. Humans are controlled by forces beyond our understanding.
In a covalent compound the bond length can be defined as. Haxton told his class that a water molecule can make 4 hydrogen bonds all of them in the same plane as the three atoms. Dissolving is best described as.
A universal solvent capable of dissolving polar and non-polar moleculos. The molecule that is best described as glucose has the chemical formula C6H12O6. O single covalent bond double covalent bond triple covalent bond ionic bond.
Humans and bacteria share a common genetic code. Humans are controlled by forces beyond our understanding. Each atom makes one hydrogen bond for a total of 3.
Humans should help in the conservation of other animal species. A polar molecule where the hydrogen is more electronegative than oxygen A good solvent capable of dissolving polar molecules and ions. This imparts partial negative charge to the oxygen atom and partial positive charge to hydrogen atoms.
Water is a source of _____ for chemical reactions in cells. The distance between two nuclei when the attraction is greater. 3 The charge is neutral and equal throughout the molecule.
Human history is determined by a series of supernatural events. 2 The oxygen end is negative relative to the end with the two hydrogen atoms. Humans should help in the conservation of other animal species.
A water molecule consists of one oxygen atom bonded to two hydrogen atoms by covalent bonds. The following molecule is best described as a. The distance between two nuclei when repulsion and attraction are balanced.
The distance between two nuclei when the repulsion is greatest. The most common example of this is water. 1 The oxygen end is positive relative to the end with the two hydrogen atoms.
Both carbon dioxide molecules and water molecules have polar bonds Why then is carbon dioxide a non polar molecule while water is a polar molecule it depends upon structure of both the molecules. The tendency of an atom to pull electrons toward itself is referred to as its _____. The distance between the two large atoms.
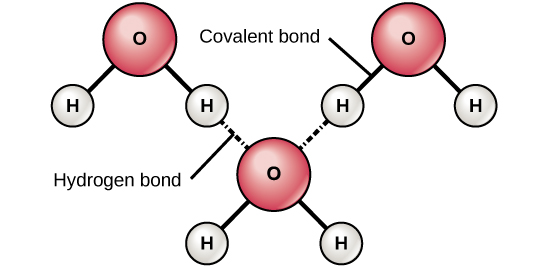
Why Life Depends On Water Biology For Non Majors I

Lesson Summary Water And Life Article Khan Academy

The Structure And Properties Of Water Introduction To Chemistry

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey
Comments
Post a Comment